UK’s first self-administered injectable could open doors
25th September 2015
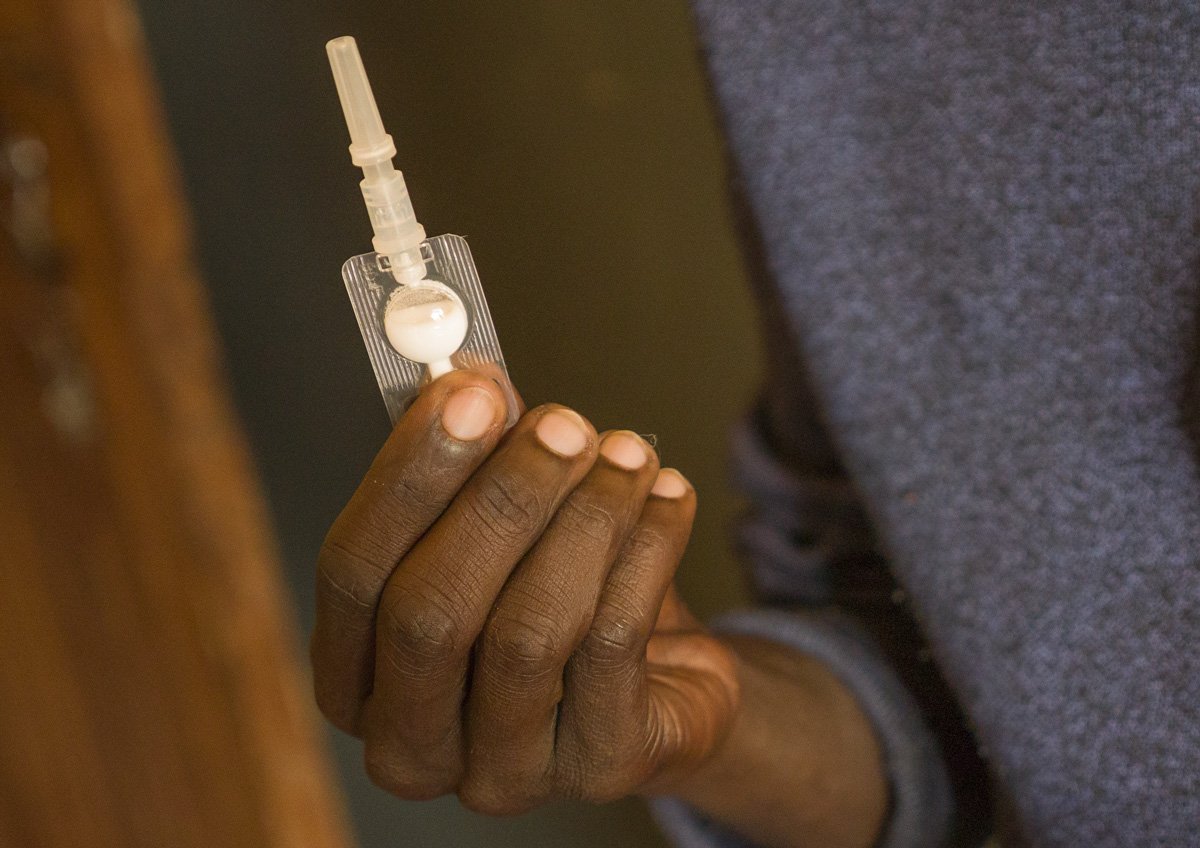
The UK market for contraceptives has received a new addition, expanding choice not just in the UK but potentially worldwide. A leading UK regulatory authority, the Medicines & Healthcare products Regulatory Agency (MHRA), has authorized self-injection of Pfizer’s subcutaneous injectable contraceptive, Sayana® Press, in that country. This update makes for the first injectable contraceptive in the UK available for self-administration. Sayana Press is packaged in the Uniject™ injection system, developed by PATH, combining one dose of the contraceptive and a single-use needle in one device. Pfizer’s announcement includes plans to seek regulatory approval of the updated Sayana Press label in a number of additional countries with unmet need and demand for injectable contraception, such as Burkina Faso, Uganda, and Senegal. Read more here.
Category: